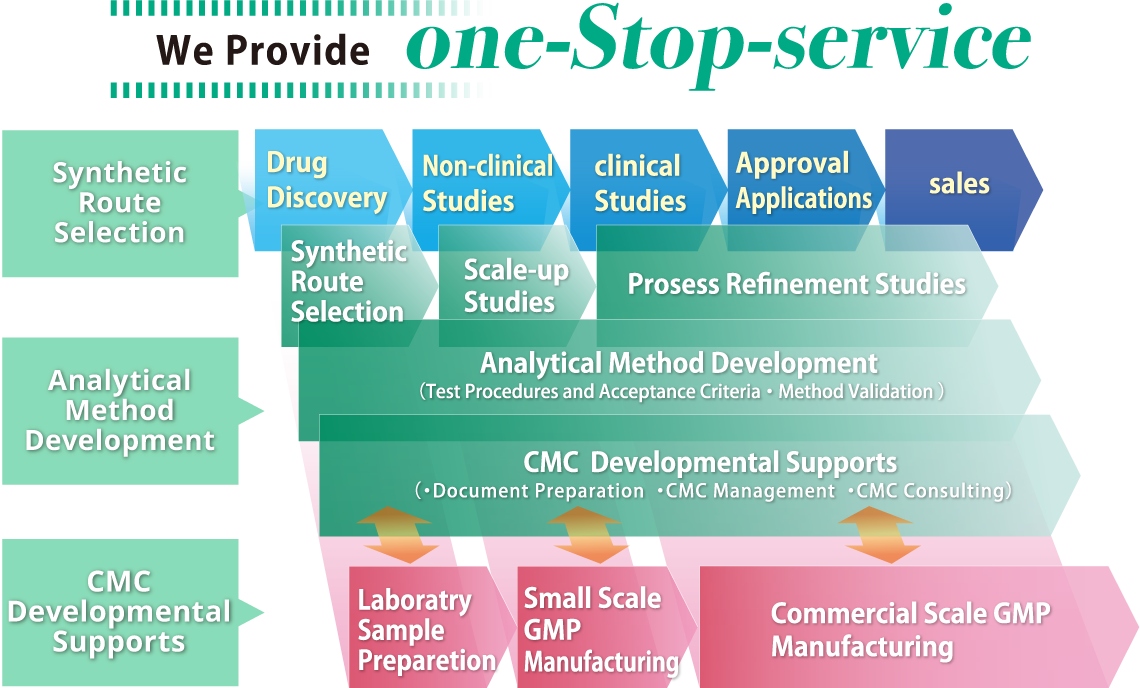
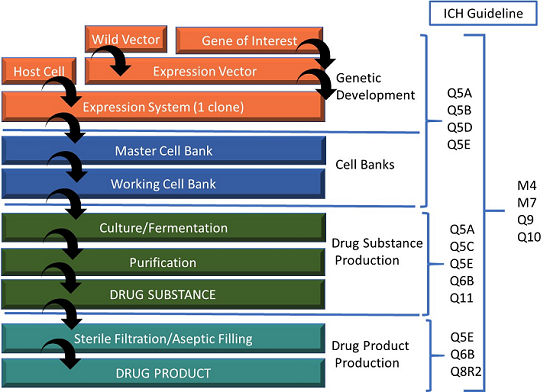
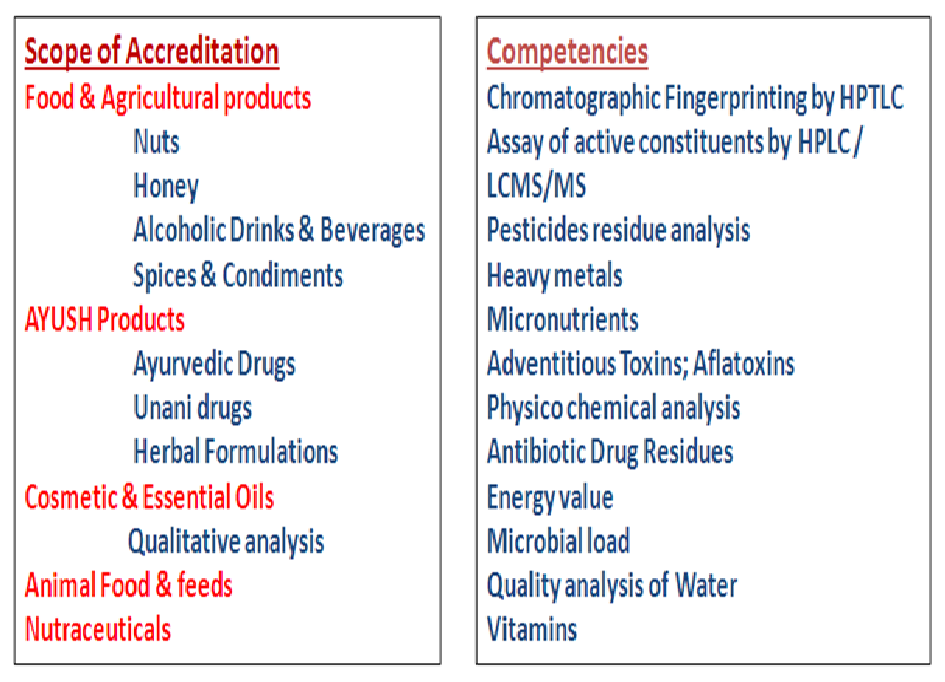
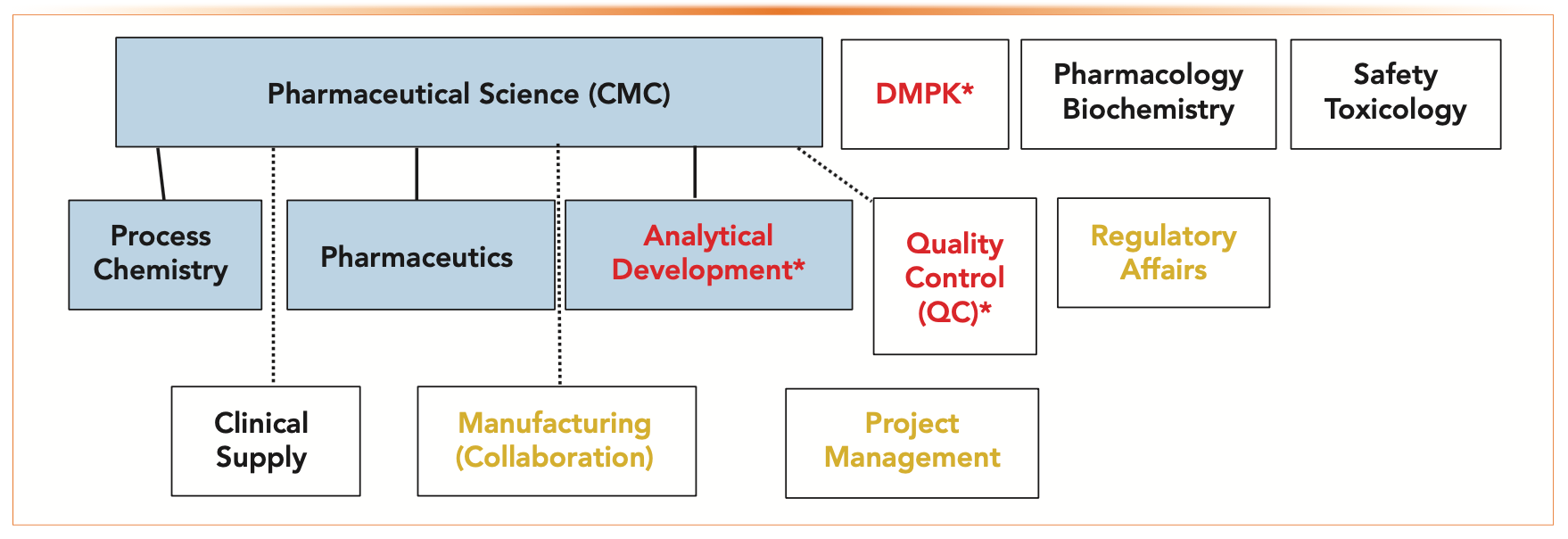
Continued Process Verification: Evolution of Biopharmaceutical Control Strategy - CMC ForumBioProcess International

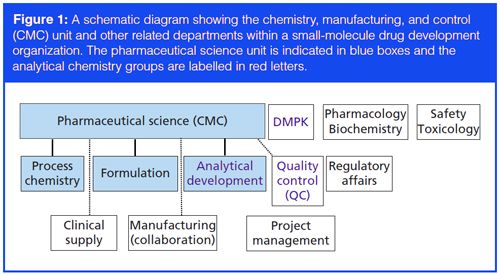
Control Strategy Expectations in Early Clinical Phase Synthetic Oncology Programs: Two Global Regulatory Case Studies | Organic Process Research & Development